NDC Number Setup
If your practice needs to add National Drug Codes (NDC's) to report
to insurance carriers, you will need to add the NDC code to the injection
procedure code(s) and use the NDC Attachment when posting procedures.
An NDC Attachment will automatically be created for procedure codes that
contain a value for the NDC Code
field or that have the NDC Required
check box selected, when they are entered in Procedure Entry.
Set up the Procedure Code(s)
Complete the following steps to set up the NDC's.
- From Tables, Procedure Code Table, Maintain Procedure Codes:
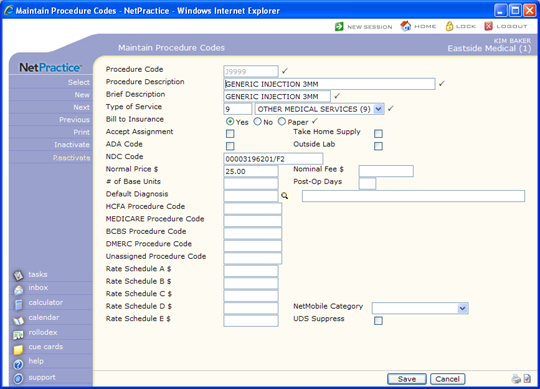
- Select an injection procedure code, select the
Requires NDC check box and type the NDC in the NDC Code
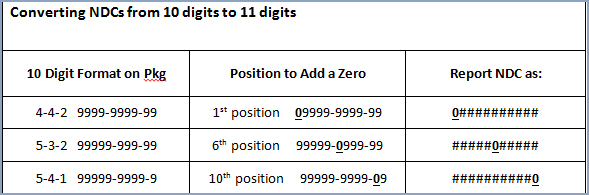
field in every applicable injection procedure. Proper billing of an
NDC requires an 11-digit number in a 5-4-2 format. Converting NDC
codes from a 10-digit to an 11-digit format requires a strategically
placed zero, dependent upon the 10-digit format. The following table
shows common 10-digit NDC formats indicated on packaging and the associated
conversion to an 11-digit format, using the proper placement of a
zero. The additional “0” is in a bold font and underlined in the following
examples:
- If you want the NDC Attachment in Procedure Entry to automatically
populate any or all of the NDC data fields, enter the data pieces
you want separated by forward slashes in the format of 'NDC/Unit of
Measure/Quantity of Medication' (NDC-11 characters, Unit of Measure-2
characters, and Quantity of Medication-up to 11 characters.
When you have completed entering the NDC information for the
injection code(s), you are ready to use the NDC
Attachment in Procedure Entry.


