
This attachment does not have any specific triggers, but is required for DMERC carriers when this Certificate of Medical Necessity is needed.
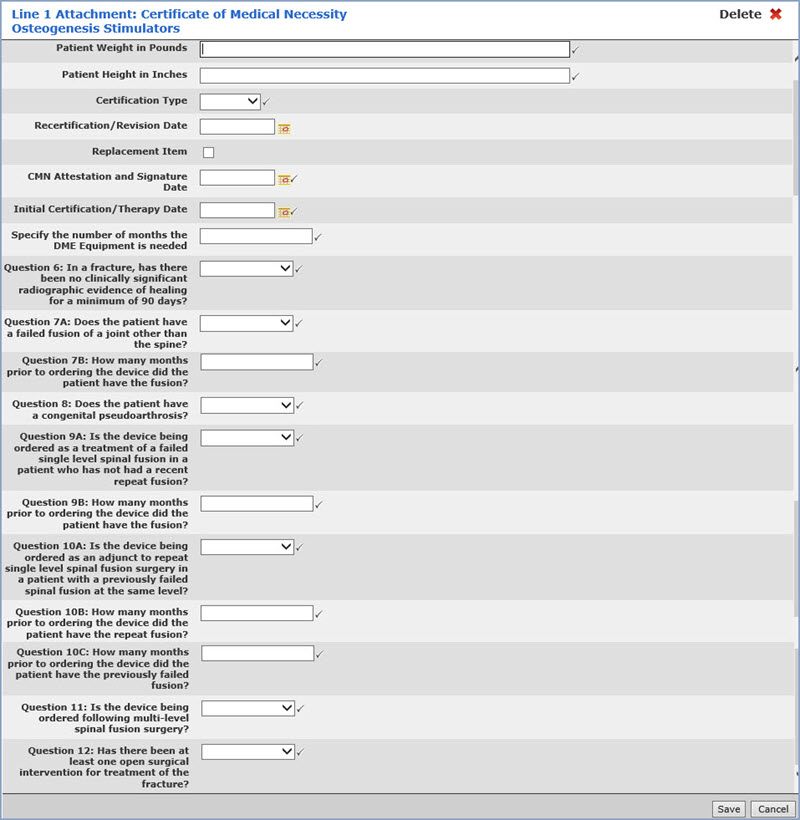
Data Field Information
| Prompt | Response | Req | Len |
|---|---|---|---|
| Patient Weight in Pounds | Enter the patient's weight in pounds. Populates Loop 2000B, Segment PAT. | 
|
7 |
| Patient Height in Inches | Enter the patient's height in inches. Populates Loop 2400, Segment MEA. |  |
4 |
| Certification Type | Select the certification type from the list. Populates Loop 2400, Segment CR3. |  |
1 |
| Recertification/Revision Date | Enter the date you want or click the calendar icon to select a date. Populates Loop 2400, Segment DTP*607. | 10 | |
| Replacement Item | If this is a replacement item, select this check box. Populates Loop 2400, Segment CRC. | 10 | |
| CMN Attestation and Signature Date | Enter the date you want or click the calendar icon to select a date. Populates Loop 2400, Segment DTP*461. | 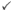 |
10 |
| Initial Certification/Therapy Date | Enter the date you want or click the calendar icon to select a date. Populates Loop 2400, Segment DTP*463. | 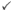 |
10 |
| Specify the number of months the DME Equipment is needed | Enter the number of months. Populates Loop 2400, Segment CR3 | 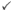 |
50 |
| Question 6: In a fracture, has there been no clinically significant radiographic evidence of healing for a minimum of 90 days | 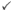 |
1 |
|
| Question 7A: Does the patient have a failed fusion of a joint other than the spine? |  |
1 | |
| Question 7B: How many months prior to ordering the device did the patient have the fusion? |  |
50 | |
| Question 8: Does the patient have a congenital pseudoarthrosis? |  |
1 | |
| Question 9A: Is the device being ordered as a treatment of a failed single level spinal fusion in a patient who has not had a recent repeat fusion? |  |
1 |
|
| Question 9B: How many months prior to ordering the device did the patient have the fusion? |  |
50 | |
| Question 10A: Is the device being ordered as an adjunct to repeat single level spinal fusion surgery in a patient with a previously failed spinal fusion at the same level? |  |
1 | |
| Question 10B: How many months prior to ordering the device did the patient have the repeat fusion? |  |
50 | |
| Question 10C: How many months prior to ordering the device did the patient have the previously failed fusion? |  |
50 | |
| Question 11: Is the device being ordered following multi-level spinal fusion surgery? |  |
1 | |
| Question 12: Has there been at least one open surgical intervention for the treatment of the fracture? |  |
1 |